Chimeric Antigen Receptor (CAR)-T cell therapy, a groundbreaking form of immunotherapy harnessing a patient’s own T cells to combat cancer, has undergone remarkable advancements over the last decade. These advancements, demonstrated through numerous successful clinical trials and regulatory approvals, have positioned CAR-T therapy as a transformative approach in cancer treatment worldwide. Given the CAR-T cells’ superior efficacy in treating hematological malignancies compared to solid cancers, CAR-T therapy targets specific markers like CD19, BCMA, and CD22. There are other ongoing clinical trials in India which are exploring the potential of CAR-T cell therapy in a broader spectrum of cancers, including acute lymphoblastic leukemia (ALL), multiple myeloma, chronic lymphocytic leukemia (CLL), head and neck squamous cell carcinoma, glioblastoma, and hepatocellular carcinoma.
The incidence of cancer in India remains high, with estimated number of incident cases of cancer in 2022 to be 14,61,427, which is 100.4 per 100,000 people. Cancer is quite common, with estimates indicating that one in every 68 men and one in every 29 women in India may develop cancer over their lifetime. Furthermore, the country witnesses about a million new cancer cases each year, accounting for roughly 8% of the global cancer burden. Despite a lower incidence rate, India has a greater fatality rate, emphasizing the critical need for sophisticated therapies. Without access to such interventions, forecasts show that the number of cancer cases in India could exceed 1.3 million by 2040. India, along with other significant global markets such as the US, EU, and China, has now emerged as a significant player in the field of CAR-T therapy.
ImmunoAct – the journey of NexCAR19
Actalycabtagene autoleucel, now marketed as NexCAR19, received marketing authorisation by Central Drugs Standard Control Organization (CDSCO) in October 2023. It became the first CAR-T cell therapy to be approved in India. The journey of NexCAR19 in India dates back to 2015 when students of Indian Institute of Technology, Bombay (IIT-B) felt the need of researching and manufacturing CAR-T cells in India. With invaluable support from esteemed institutions like the NIH Clinical Centre and NCI’s Center for Cancer Research, they were able to design a CAR-T cell therapy that was safe, effective and could be manufactured in India.
In 2018, their endeavors came to fruition with the establishment of ImmunoACT, a company incubated at IIT Bombay poised to redefine cancer treatment in India. With IIT-Bombay serving as its dedicated research and development partner and the esteemed Tata Memorial Centre (TMC) as its collaborator for clinical trials, ImmunoACT embarked on a journey to pioneer cellular therapy. In August 2019, IIT-B filed a patent application WO2019159193, with a priority date of 2018, protecting the CAR construct of their brainchild, NexCAR19. The clinical trials of NexCAR19 commenced in mid-2021 at the Tata Memorial Centre (TMC), culminating in a historic milestone on June 4th, 2021, with the first dose of CAR-T cell therapy administered at the Bone Marrow Transplant unit at ACTREC, TMC in Mumbai. Subsequently, almost 30 months later, positive safety and efficacy profiles of NexCAR19 led to its approval in India in October 2023 for treatment of relapsed/refractory B-cell lymphomas and leukemia. This marked a significant milestone, showcasing India’s embrace of advanced cancer treatments on the global stage.
To support this journey, ImmunoACT initially received seed funding from IIT-Bombay and later garnered support from philanthropic organizations such as the Wadhwani Foundation in Bangalore and Tata Trusts in Mumbai. Additionally, a support of ₹19.15 crore for conducting the first and second phase clinical trials on humans came from the Biotechnology Industry Research Assistance Council (BIRAC), an interface bridging industry and academia under the Indian Department of Biotechnology (DBT). Subsequently, Laurus Labs played a pivotal role as an early investor in ImmunoACT, contributing over $18 million to facilitate the scaling of research and development, as well as commercialization efforts. Presently, Laurus Labs holds approximately a 34% stake in ImmunoACT.
One of the hurdles in cell and gene therapy (CGT) approvals in India is the regulatory framework for evaluating CGT products. India is the largest generic manufacturer globally. Thus, a large portion of regulatory framework revolves around proving safety and efficacy equivalence. However, the complex nature of CGT products, especially NexCAR19, called for a much-needed knowledge sharing between various regulators and stakeholders as well as improvising the existing regulatory framework. Review Committee on Genetic Manipulation (RCGM) and the Indian Council for Medical Research (ICMR) provided scientific inputs on what these guidelines should be from their perspectives, which led to the CDSCO finalizing its guidance.
Another hurdle in the approval of such products is the supply chain. Most of the conventional drug products follow off-the-shelf supply chain profile. But, CAR-T cells follow a circular supply chain, that is, collecting patient’s cells, sending them to manufacturing unit, and then sending it back to hospital for infusing them back to the patient. This is a novel process. During its journey, NexCAR19 sponsors had to train all involved healthcare providers as well as staff in the manufacturing units at different levels in order for its successful administration. Understanding of these commercial operations between the hospitals and ImmunoACT was explained in the process of queries and responses over the course of four to five months.
Lastly, affordability of NexCAR19 is another battle that ImmunoACT had to fight. ImmunoACT knew that if the intellectual property (IP) that protects the CAR construct, vectors, and plasmid is not owned end-to-end and is in-licensed from major markets, a higher licensing fees needs to be paid and thus, may lead to increased prices. Thus, ImmunoACT (IIT-Bombay) maintained the ownership of its IP and thus, led to affordable pricing of NexCAR19. But that’s not all. The total treatment cost was indirectly further cut down by humanizing the CAR construct. This humanization led to decreased incidence of the most common adverse event involved in CGTs, such as cytokine release syndrome and neurotoxicity syndromes. Lesser the adverse events, lesser is the total treatment cost to the patients.
With that, ImmunoACT has partnered with around 20 hospitals throughout India. These hospitals are: Tata Memorial Centre, Apollo hospitals, Kokilabena Dhirubhai Ambani research institute, Sahyadri hospitals, Fortis hospitals, Jaslok hospital, SMBT hospital, P.D Hinduja hospital, Ruby Hall clinic, Amrita hospital, MAX healthcare, Rajiv Gandhi cancer institute and research centre, BLK-MAX Super Speciality hospital, WIA cancer institute, Continental hospitals, KIMS hospital, AIG hospital, Basavatarakam Indo-American Cancer hospital and research institute, St. John’s hospital, and Cytecare hospitals.
As of February 2024, a total of 15 patients have been administered with NexCAR19 in India. Three of them have successfully achieved cancer remission. One patient has been successfully declared cancer-free. The patient, Dr (Col) VK Gupta, a Delhi-based gastroenterologist, took this therapy by paying just Rs 42 lakh which would have otherwise cost him Rs 4 crore abroad. Although it’s very early to decide on the success of the therapy, it sure shows a lot of promise in otherwise a very complicated disease area.
The journey of NexCAR19 has been summarized in figure 1.
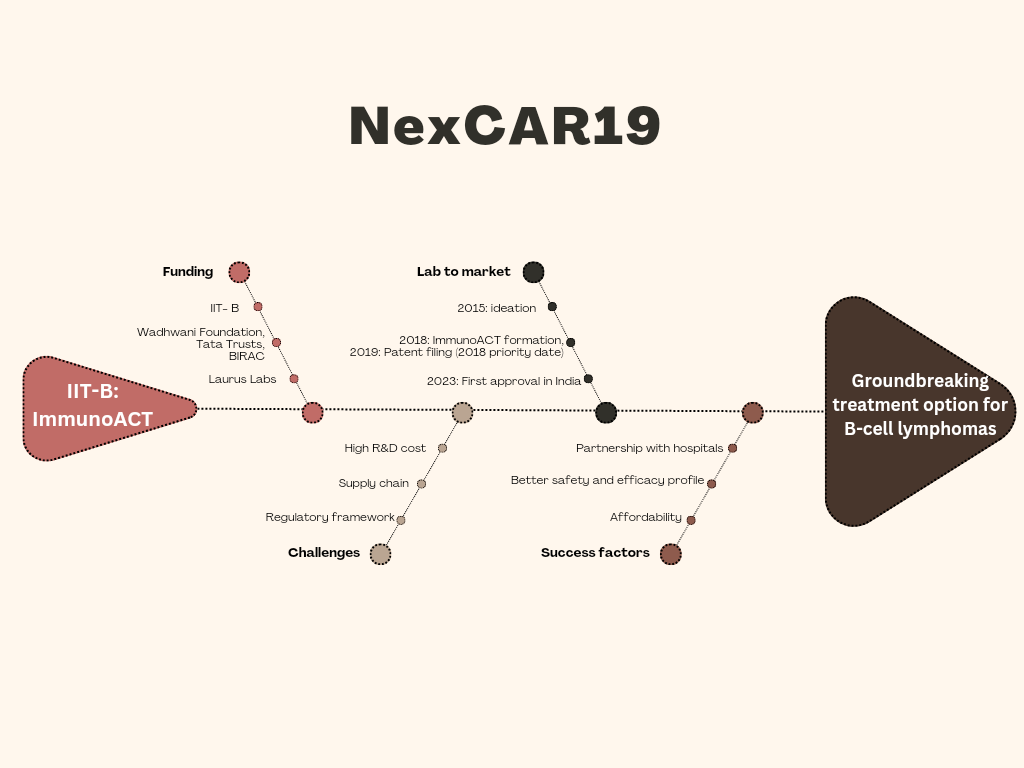
Figure 1: The journey of NexCAR19 to becoming a groundbreaking treatment option for B-cell lymphomas in India.
Other CGT pioneers in India
NexCAR19 is not the only development in the CGT space in India. There are many other collaborations between industry entities and academic institutions in India that have been instrumental in driving innovation in the field of gene therapy. These collaborative endeavors between universities and companies not only ensure adequate funding for research and development but also offer opportunities for exclusive commercialization, thereby creating a mutually beneficial scenario for all parties involved (Figure 2).
Some of the advances to look forward to in the Indian CGT space are follows:
- Indian Institute of Technology (IIT) Kanpur’s partnership with Reliance Life Sciences Pvt. Ltd., since March 2023, exemplifies the synergy between academia and industry in advancing gene therapy technologies, particularly in addressing genetic eye diseases. This gene therapy technology from IIT Kanpur will be further developed as an indigenous product by Reliance Life Sciences. This patented technology alters the genetic makeup of an organism to address a genetic condition.
- In June 2023, Indian Institute of Technology Kanpur (IITK) and Laurus Labs collaborated through a Memorandum of Agreement (MOA) to introduce innovative gene therapy solutions to the market. This partnership signifies a significant shift in the landscape of bioengineering innovation in India. Per the terms of the MOA, IIT Kanpur will transfer several gene therapy assets to Laurus Labs and Laurus Labs, in turn, will provide research funding to support the advancement of these assets through pre-clinical development and subsequent clinical trials. Furthermore, Laurus Labs will lead the commercialization efforts for these cutting-edge products, not only in India but also in emerging markets. Additionally, as part of the collaboration, Laurus Labs will establish a state-of-the-art Good Manufacturing Practice (GMP) facility at IIT Kanpur’s Techno Park, thereby enhancing the production capabilities for gene therapy products. This initiative will enable Laurus Labs to actively participate in the Contract Development and Manufacturing Organization (CDMO) business for cell and gene therapy, leveraging the advanced infrastructure of the GMP facility.
- Eyestem, a Bengaluru-based company, is developing Eyecyte-PRP and Eyecyte-RPE. Eyestem’s collaboration with the National Institute of Immunology, New Delhi in developing Eyecyte-PRP, underscores the collaborative efforts towards developing novel therapies for eye-related conditions.
- In August 2019, the country’s largest biotech, Biocon, announced the formation of Immuneel Therapeutics, a startup with the US founders, in order to bring CAR-T cell therapies to India. Immuneel has exclusive rights to ARI-0001/IMN-003A (a CD-19 CAR-T cell therapy- Varnimcabtagene Autoleucel) from Hospital Clínic de Barcelona and Institut d’Investigacions Biomèdiques August Pi i Sunyer in Spain to develop, manufacture and commercialize in India via technology transfer. Immuneel has begun patient dosing in a CAR-T phase II trial named “IMAGINE” in India.
- Regrow biosciences is developing products such as Ossgrow, Cartigrow and Uregrow targeting indications like osteonecrosis, cartilage repair and urethral stricture, respectively.
- The Centre of Cell and gene therapy of Intas Pharmaceuticals shows a strong pipeline with 6 AAV based gene therapy and 3 LV based cell therapy programs.
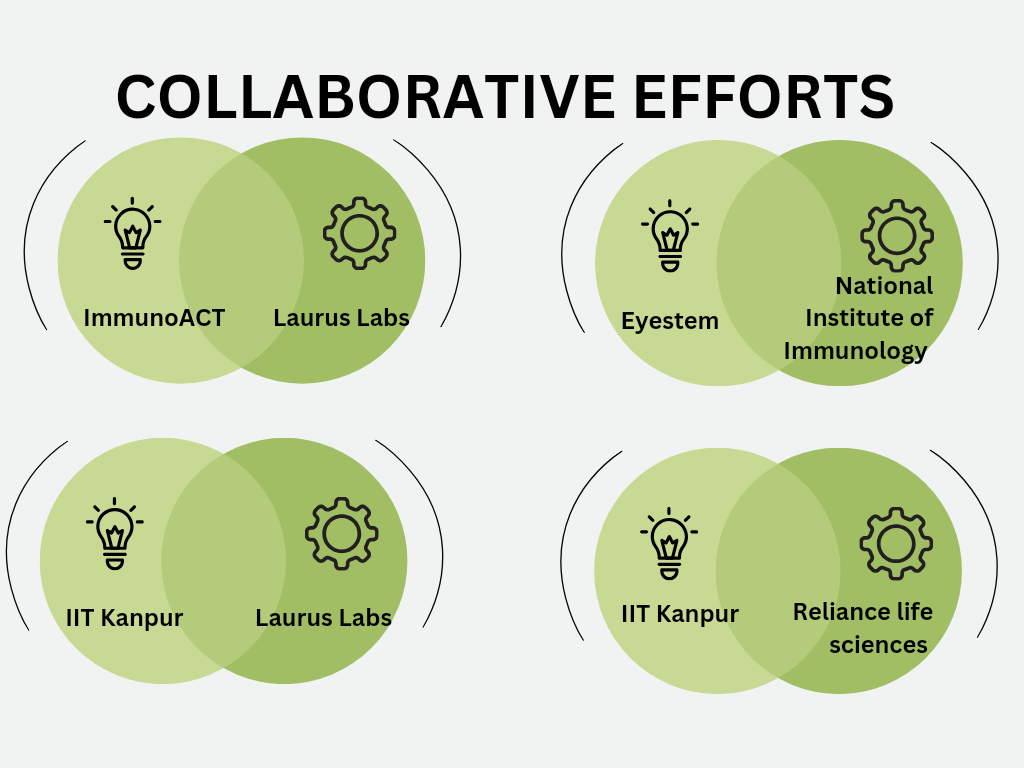
Figure 2: Collaborative efforts between industry entities and academic institutions in India in the CGT space.
The IP landscape of these therapies also look promising, thus helping the overall research and development in the CGT space. Some of the patent filings of these sponsors include:
| 1 | Eyestem | US2022162563, US11168301 |
| 2 | Stempeutics | US10471101, WO2016001839 |
| 3 | Regrow biosciences private ltd | WO2023275880, US10577587 |
| 4 | Intas Pharmaceuticals Ltd | WO2022029543, WO2022107058 |
| 5 | IIT Kanpur | WO2020099956, WO2020058786 |
| 6 | Immuneel | EP2022062374 |
The strong IP portfolio helps the academics and companies gain a strong footprint and later leverage it. Considering the increasing research in this field, it would be interesting to see more patent filings and grants in coming years.
The way forward
To make cell therapies more affordable, innovation and holistic development are crucial. India lacks an ecosystem for high-quality raw materials and high-technology services, which is essential for the biotech industry. The cost of manufacturing and development costs, including preclinical, drug, IP generation, and clinical development, are significant. One of the ways to address this is having an indigenous intellectual property. India has developed deep tech innovation, enabling the development of local IP and bringing innovative therapies to underserved populations. Other ways to reduce costs include using abandoned lentiviral vectors which has previously proven efficacy in clinical trials, point-of-care manufacturing such as the in-licensing done by Immuneel, using biomarkers to identify patients that would respond to the treatment, and setting up manufacturing units in low-labor-cost areas. These strategies aim to reduce the overall cost of cell therapies and improve the overall healthcare system. With that, the Indian regulators are already paving way to address the issues in regulatory frameworks and enhance guidelines to help foster innovation in the cell and gene therapy areas.
References:
- https://www.cellandgene.com/doc/paving-the-way-for-india-s-first-domestic-car-t-approval-0001

