An Usher of Immunology Biosimilars in the Next 5 Years?
Biosimilars are biologic medicines that are highly similar to existing licensed biologic products with no clinically meaningful differences in terms of safety and efficacy. Unlike generics, biosimilars are manufactured using complex living organisms and other natural resources. In the absence of a defined structure and due to variations in the growth conditions of genetically engineered cells, biosimilars always have slight modifications and are not exact copycats of the reference biological product. They are also generally 20-30% cheaper than the original biologics as they enter the market once the patent on the original biologic has completed it’s 20-year term.
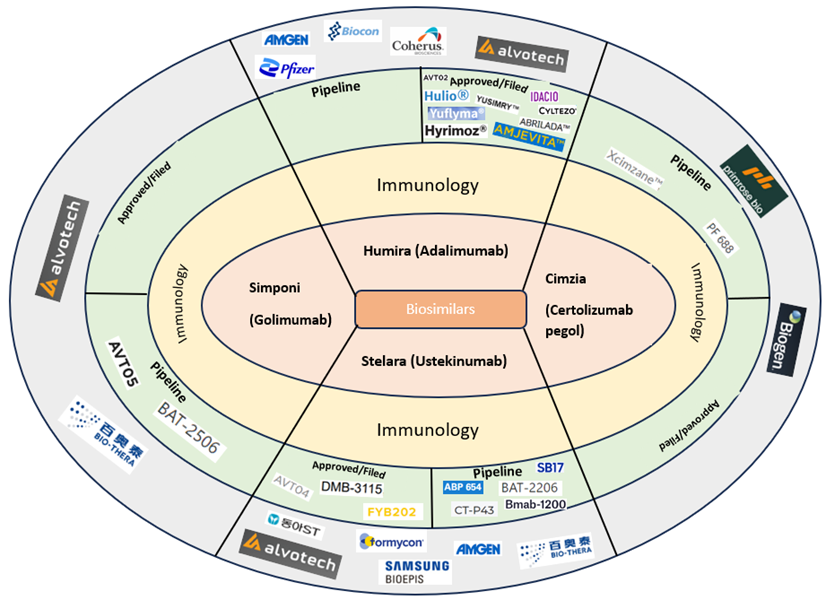
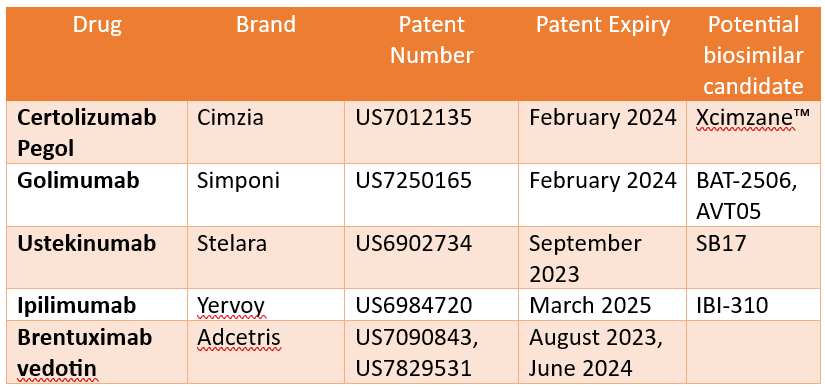
Many biologics are estimated to lose patent protection in the next five years which will create opportunities for biosimilars to enter the market. This in turn will increase market competition (Figure 1).
Factors like:
1. Growing prevalence of inflammatory and immune-mediated chronic diseases,
2. increased acceptance, and approvals of biosimilars by regulatory bodies,
3. new investors waiting to invest in this sector
are all the factors that will fuel the growth of biosimilars market.
To speed up the biosimilar development, it is required to streamline the clinical studies. Most biosimilar approvals include pharmacokinetic (PK) similarity data and a comparative clinical study which are generally time-consuming, costly, and needs a large number of participants.
A more streamlined approach may be to submit PK and pharmacodynamic (PD) similarity data alongside comparative safety and immunogenicity data. Investigators can collect PK and PD data from shorter, less costly studies, often enrolling healthy participants.
With more and more biopharmaceutical companies targeting biosimilar candidates for biologics nearing their patent expiry, Figure 2 summarizes the status of approved biosimilars and candidates in clinical trials for immunology blockbuster biologics.