A Detailed Analysis of Patent Term Extensions Expiring in the U.S. between 2023-2028
A drug manufacturer can enjoy monopoly in the market till 20 years from the first filing date of a patent application. However, owing to complexity of the regulatory process of the U.S. Food and Drug Administration (FDA), a significant time is lost in getting the drug approved and the patentee is unable to reap the benefits of the granted drug patent until the drug is commercially marketed.
Under the 1984 Drug Price Competition and Patent Restoration Act, also known as the Hatch-Waxman Act (The Act), a compensatory mechanism called patent term extension (PTE) was made available to the patentees to restore the lost patent term, up to a maximum of 5 years, while they waited to get the regulatory approval. Under all circumstances, the total patent life for the drug product with the patent term extension cannot exceed 14 years from the drug product’s first approval date.
Active ingredients of new drugs or human biologic products, medical devices, food additives, or color additives, and animal drug products are eligible for patent term extension. Only one patent gets extended for a regulatory review period for any drug product. If more than one application for extension is filed by a single applicant which seeks the extension of the term of two or more patents based upon the same regulatory review period, the certificate of extension of patent term, if appropriate, will be issued upon the application for extension of the patent term having the earliest date of issuance of those patents for which extension is sought.
A total of 610 PTE applications were filed in the last 5 years (i.e. starting from 4th October 2018), a majority of which cover the gene therapy products such as HEMGENIX, ZOLGENSMA, VYJUVEK, ROCTAVIAN, and OMISIRGE, monoclonal antibodies such as POTELIGEO, LUMOXITI, POLIVY, ENHERTU, as well as SGLT2 inhibitors like BRENZAVVY, BEXACAT, and INPEFA.
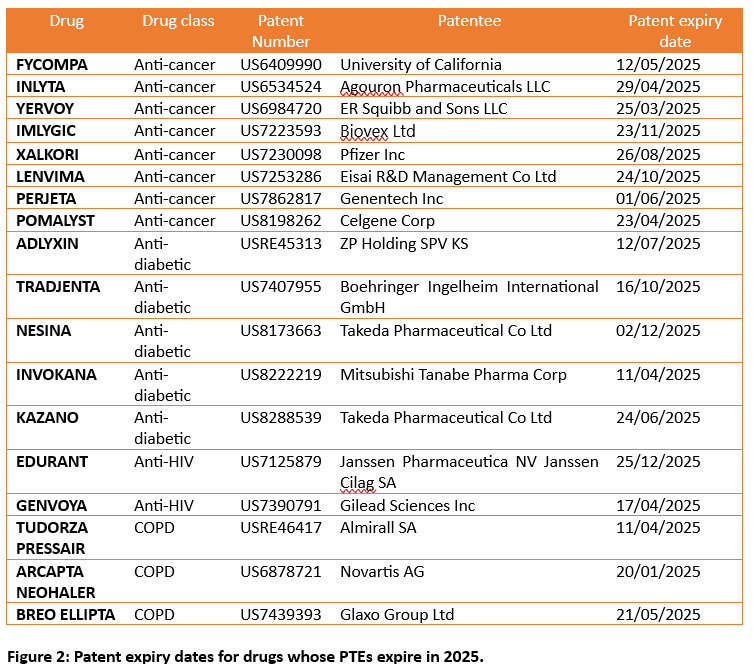
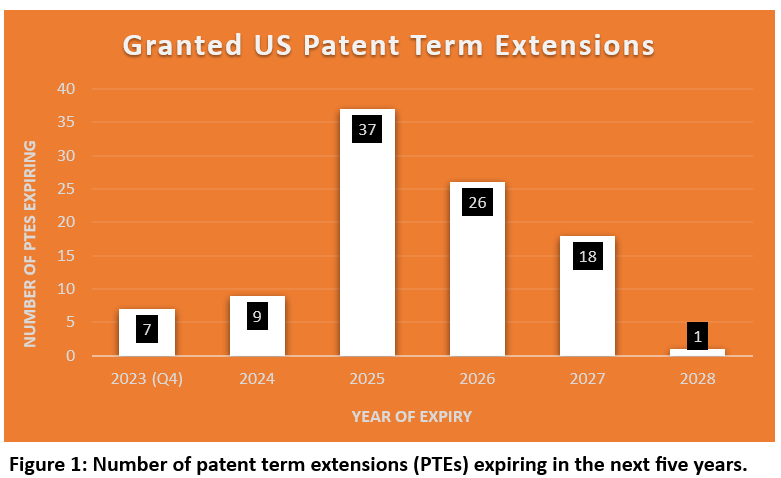
A detailed analysis of the PTEs granted in the last 5 years indicate that 2025 is the year to keep a close eye on as 37 PTEs (Figure 1) covering:
- anti-cancer drugs – FYCOMPA, INLYTA, YERVOY, IMLYGIC, XALKORI, LENVIMA, PERJETA, POMALYST
- anti-diabetic drugs – ADLYXIN, TRADJENTA, NESINA, INVOKANA, KAZANO
- anti-HIV drugs – EDURANT, GENVOYA
- COPD drugs – TUDORZA PRESSAIR, ARCAPTA NEOHALER, and BREO ELLIPTA are set to expire.
Takeda Pharmaceuticals, Pfizer Inc, and Janssen Pharmaceuticals will lose patent protection in 2025 for many of their blockbuster drugs (Figure 2). This will lead to many more opportunities for market consolidation and generics entering the market, thus making the expensive anti-cancer and anti-diabetic drugs available to the patients in need at affordable prices.